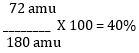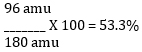% Composition: percentage by mass of each atom in a formula or compound.

Example: Determine the % composition of each atom in glucose, C6H12O6
Steps to Solve:
1. List all elements in the compound for which you would like to determine the percentage composition.
C:
H:
O:
2. Calculate the TOTAL mass of each element in the formula in small “chunks”.
C: (6 atoms of C) x (12 g) = 72 g
H: (12 atoms of H) x (1 g) = 12 g
O: (6 atoms of O) x (16 g) = 96 g
3. Determine the formula weight (by adding all mass of each element)
72 g + 12 g + 96 g = 180 g
Related Byte: Calculating Formula Weight
4. Divide each element’s TOTAL mass by the formula weight, then multiply by 100 to attain the percentage.
Double check: the sum of all percentages (40% + 6.7% + 53.3%) = 100%!