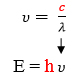Maxwell, Plank, and Bohr’s equations are all interrelated. Constant values are highlighted in red. A variable from one equation can be plugged into another such that the three equations can all be used together to relate wavelength, frequency, energy, and shell number.
Example: What is the wavelength of a electron transitioning from n= 3 to n= 1? Is this electron emitting or absorbing energy? Explain.
Given:
- ni = 3
- nf = 1
Constants:
- c = 3.00 x 108 m/s
- h = 6.63 x 10-34 J s
- RH = 2.18 x 10-18 J
Solve:
PART I: Solve for energy
PART II: Combined Maxwell and Plank equations and solve for wavelength using energy value found in part I.