Schrodinger wrote an equation that described both the particle and wave nature of the electron. This is a complex equation that uses wave functions to relate energy values of electrons to their location within the atom. A more qualitative analysis can at least describe
Wave function (ψ) describes:
- energy of e- with a given
- probability of finding e- in a volume of space
In addition to being mathematically complex, another downfall of Schrodinger’s equation is that it can be solved exactly for only the hydrogen atom—since it contains 1 lone electron. For all other elements, the ψ must be approximated and best that can be done to find ψ2, or the area of highest probability of finding an electron.
Schrodinger’s wave equation takes into account 4 quantum numbers which are further discussed below:
- Principal Quantum # (n):
- Represents the shell #;
- Possible values are n= 1, 2, 3, 4…7
- Refers to the 7 rows/periods on the periodic table
- Angular Momentum Quantum # (l):
- Represents the subshell and shape of the orbital
- Has numerical values of 0, 1, 2, and 3 which also refer to letters as below
- Possible values:
- 0 1 2 3
s p d f - Therefore, you can refer to a subshell by its numerical or letter value (i.e., l=2 or d)
- Refers to one of four sections of the periodic table as illustrated in the color-coded table below:
NOTE: “s” and “p” sections have a quantum number value equal to the row number or “n,” while the “d” section has a value of “n-1” and the f section as a value of “n-2.”
This means that the principle quantum number for an electron on the 6th row would be 6s, 6p, (6-1) or 5d, and (6-2) or 4f.
- Magnetic Quantum # (ml):
- Refers to orbital #
- Possible values are ml = 0, ±1, ±2, ±3
- No reference to periodic table but orbitals are drawn as diagrams (a box represents each orbital) and electrons are placed inside them.
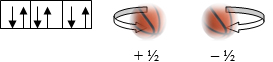
- Magnetic Spin Quantum # (ms):
- Refers to electron spin
- Electrons are drawn as arrows
- Since like charges of electrons in a pair repel, electrons spin away from one another or have opposite spin directions, noted as a + ½ or – ½ here.
- Each arrow is an electron, point in opposite directions
- Each value (1 electron) refers to one block or square on the periodic. A good analogy is like rolling dice and moving spots on a board game.
Possible Quantum Numbers Diagram for Fourth Shell (n=4):