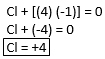Oxidation-Reduction Reactions: electron transfer reactions; one substance loses electrons while the other gains electrons; charges will change; typically exothermic and gas producing.
- Oxidation: process of losing electrons; results in the charge of atom increasing
- Reduction: process of gaining electrons; results in the charge of the atom decreasing
The A, B, C’s of Redox Reactions:
1. Redox reactions can take the form of composition, decomposition, exchange/displacement.
2. They typically involve a reaction between two metals (and one of their respective salts) or a metal and hydrogen.
3. Combustion reactions are typically redox reactions.
4. For each element involved in a redox reaction, you should determine its charge, or oxidation number, before and after the reaction.
Oxidation Number: same as charge on the atom; represents the number of electrons lost or gained; an atom can have more than one form or oxidation number (ie Cl+7, Cl+5, Cl-1)
5. Identify which element’s charge increased. That atom has been oxidized. The atom which has been reduced will see a decrease in its charge.
- Oxidized element: charge increases; also called the reducing agent
- Reduced element: charge decreases; also called the oxidizing agent
6. The changes in charge or oxidation number are equal to the number of electrons lost/gained.
NOTE: Since electrons are matter, they must be conserved; therefore, in any redox reaction, the number of electrons lost must equal the number of electrons gained. We achieve this by ensuring the chemical equation is always balanced.
Guidelines for Determining Oxidation Number:
- Atoms in their original form and on their own have an oxidation number of zero.
- Ex. O2, Zn, Cl2, S8, Cu, H2
- Monoatomic ions have an oxidation number equal to their charges. If an ion already has a charge listed, that is its oxidation number.
- Ex. Na+ = +1
- Ex: Al3+ = +3
- Hydrogen has an oxidation number of +1 (except when bonded to a metal in which case it has a -1 charge).
- Oxygen has oxidation number of -2 (except in peroxides H202 where its oxidation number is -1)
- Fluorine is always -1. Group I metals are +1 and group II metals are +2
- If a polyatomic ion appears in the same form on both sides of reaction, then their oxidation numbers do not change.
- All other atoms’ oxidation numbers must be calculated using the known oxidation numbers of the other atoms to solve for the unknown atom.
- The sum of all oxidation numbers in a compound should equal the net charge on the compound. Neutral atoms should have charges adding up to zero.
NH4+ : all oxidation numbers should add up to +1
H2O: all oxidation numbers should add up to zero. Water is neutral.
Ex. NH4+
Using Rule #3 above, “H” is +1. Therefore, we solve for the oxidation number of “N,” the unknown. The sum of 1 N and 4 H atoms should equal +1, the charge on ammonium.
Ex. ClF4
Using Rule #5, we known that “F” is -1. Therefore, we solve for the oxidation number of “Cl.” Since ClF4 is neutral, the sum of 1 Cl and 4 F atoms should equal zero.
HINT: Think of these problem like algebraic equations, where the unknown element is “x.”