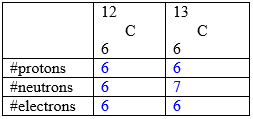
Isotopes: atoms of the same element with the same #p but different #n (and thus different atomic mass) are called isotopes.
Since isotopes have different masses, the average atomic mass of all isotopes of the element is listed on the periodic table, hence the decimal values. Average masses are calculated using the percentage of abundance of each isotope; therefore, the more abundant the isotope, the more it will influence the average mass.
Example: Boron-10 (10.01294 amu) and Boron-11 (11.00931 amu) have percentage abundances of 19.91% and has 80.09%, respectively. What is the average isotopic mass of boron?
Solution:
Convert all % to fractions by dividing by 100. Then multiply the mass by the fraction for EACH isotope. The sum of all fractions of each isotope is the average isotopic mass.
10.01294 amu x 0.1991= 1.994 amu
11.00931 amu x 0.8009= 8.817 amu
1.994 amu + 8.817= 10.81 amu (same as periodic table)


Pingback: Chemistry Fundamentals Uploaded | ChemistryBytes.com