Dilution: process of converting a more concentrated solution into a less concentrated solution by adding additional solvent.
Dilutions are performed when a less concentrated solution is needed, particularly when using a premade or stock solution, such as a concentrated acid.
The following equation is used to solve dilution problems:
M1V1 = M2V2
Where:
| M1 = molarity of first (stock) solution | M2 = molarity of second (dilute) solution |
| V1 = volume of first solution | V2 = volume of second (dilute) solution |
Example: What is the molarity of a 200 mL solution made from 50 mL of 8M stock solution of HCl?
Given:
- M1 = 8M
- V1 = 50mL
- M2 = ?
- V2 = 200 mL
Solve:
| M1V1 = M2V2 |
| (8M) (50mL) = (M2)(200mL) |
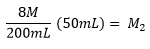 |
| 2 M = M2 |

