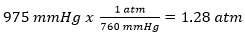In order to understand how pressure can affect other properties of a gas, you need to know more about the different pressure units used.
There are 3 Units of Pressure we will use in chemistry:
- Universal unit: atm (atmosphere)
- mm Hg (millimeters of mercury)
- Torrs (named after Torricelli, an Italian scientist)
- FYI: SI unit is pascal (Pa)
Think of a barometer (Toricelli’s work) like a thermometer for pressure. The higher the reading on the barometer, the higher the pressure. Of course, like some thermometers, barometers use the height of mercury to relay a measurement.
Since pressure is read in millimeters of mercury, we use that as a unit : mmHg. To credit Torecelli, we also call each mmHg, a Torr.
Hence: 1 mm Hg = 1 Torr
However, the more commonly used unit for pressure is the atmosphere, or atm. So we need a way to convert between the units. Each 1 atm = 760 mmHg or 760 Torrs.
Use simple dimensional analysis and you can convert between all three units using conversion factors:
1 atm = 760 mm Hg= 760 torrs
Problem: Convert 975 mmHg to atm.