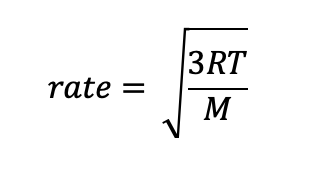
Graham’s Law of Effusion describes the relationship between a gas’ rate of effusion to be
Rate α Temperature
Rate α 1/M
a) inversely proportional to a gas’ molecular mass
- Simply put, a heavy gas moves slower than a lighter one.
b) directly proportional to a gas’ temperature
- At higher temperatures, gas molecules have more kinetic energy and therefore greater motion, hence the faster rate of diffusion.
Graham’s Law can take different forms depending on the variables being compared, for example if two gases’ rates are being compared with respect to just their molecular masses.
Below is a common form of the equation with takes into consideration both the temperature and molecular mass of a single gas.